If you need help with a LunaGens product, you can contact customer support by phone or email. The company's headquarters is located in the United States.
LunaGens (LUNG) phone number.
LunaGens can be reached at (855) 873-9279.
LunaGens (LUNG) support.
LUNG has announced that it will be supporting the LunaGens blockchain platform. This will allow developers to build applications on the LUNG platform and then use the LunaGens token to pay for transactions on the platform.
LunaGens is a blockchain platform that allows developers to build applications on the platform and then use the LunaGens token to pay for transactions on the platform. The LUNG token will be used to reward users for their participation in the platform, and it will also be used to pay for services on the platform.
LunaGens (LUNG) customer care.
If you have any questions about your LunaGens order, please contact our customer care team at 1-855-LUNAGENS (1-855-586-4357). We are available from 9am to 6pm EST Monday through Friday.
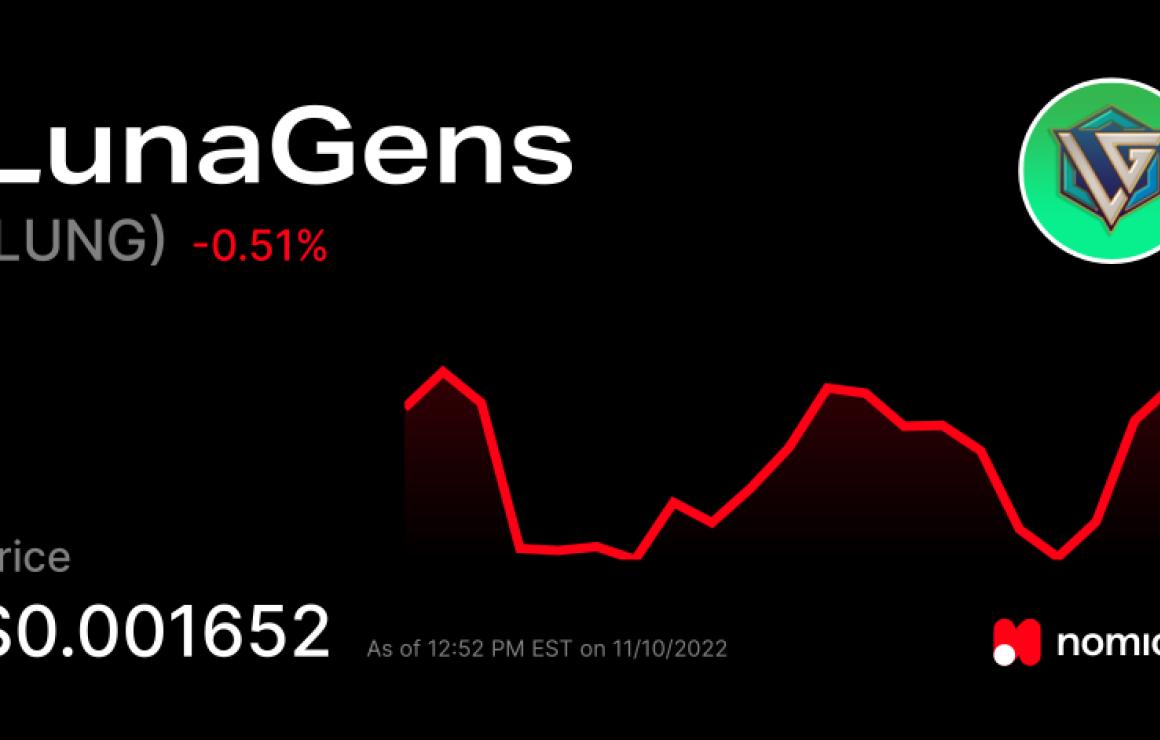
What is LunaGens (LUNG)?
LunaGens is a global network of investors, entrepreneurs and scientists who are passionate about the potential of blockchain technology. We believe that blockchain has the potential to revolutionize the way we process and store data, and we are committed to helping make this happen.
LunaGens was founded in 2017 by a team of experts in blockchain technology and data processing. Our mission is to help bring blockchain technology to the mainstream by providing access to our unique network of investors, entrepreneurs and scientists, and by working with partners to develop innovative applications using blockchain technology.
LunaGens (LUNG) headquarters.
LunaGens is a biotech company that specializes in the development of gene therapies for chronic diseases. The company was founded in 2011 by scientists from the Perelman School of Medicine at the University of Pennsylvania.
LunaGens' lead product is a gene therapy for chronic obstructive pulmonary disease (COPD). The therapy is based on a technology developed by the company and licensed from the University of Pennsylvania. The therapy is expected to be the first gene therapy to be approved by the U.S. Food and Drug Administration (FDA).
In September 2017, LunaGens announced that it had completed enrollment in a Phase III clinical trial of its gene therapy for adult patients with cystic fibrosis. The trial is expected to enroll up to 350 patients.
LunaGens has also developed gene therapies for sickle cell disease and hemophilia. The company has partnered with Pharmaceutical giant GlaxoSmithKline to develop gene therapies for these conditions.